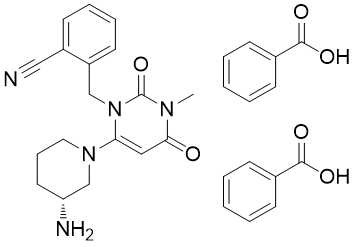
850649-62-6 Alogliptin Benzoate C25H27N5O4 691-730-4 Antidiabetic
-
High Light
Antidiabetic Pharmaceutical Intermediates
,850649-62-6 Alogliptin Benzoate
,C25H27N5O4 Alogliptin Benzoate
-
Product NameAlogliptin Benzoate
-
SynonymsALOGLIPTIN(SYK-322) BENZOATE;(R)-2-((6-(3-Aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)benz;2-[[6-[(3R)-3-aMino-1-piperidinyl]-3,4-dihydro-3-Methyl-2,4-dioxo-1(2H)-pyriMidinyl]Methyl]-Benzonitrile Monobenzoate
-
CAS850649-62-6
-
MFC25H27N5O4
-
MW461.52
-
EINECS691-730-4
-
Purity99%
-
Place of OriginChina
-
Brand NameRUN
-
CertificationIOS9001
-
Model NumberRUN-Z
-
Minimum Order Quantity10g
-
Priceinquiry
-
Packaging Details10g 50g 100g 500g 1kg
-
Delivery Time3-7 days
-
Payment TermsT/T, MoneyGram, BTCcoin
-
Supply Ability1kg --100kg
850649-62-6 Alogliptin Benzoate C25H27N5O4 691-730-4 Antidiabetic
SECTION 1: Basic information
| Alogliptin benzoate Basic information |
| Indications and Usage Mechanisms of Action Adverse reactions |
| Product Name: | Alogliptin benzoate |
| Synonyms: | (R)-2-((6-(3-aMinopiperidin-1-yl)-3-Methyl-2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)Methyl)benzonitrile benzoate;Alogliptin benzoate 2-[[6-[(3R)-3-Amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]benzonitrile benzoate;Alogliptin(Alogliptin benzoate;2-[6-[3(R)-AMinopiperidin-1-yl]-3-Methyl-2,4-dioxo-1,2,3,4-tetrahydropyriMidin-1-ylMethyl]benzonitrile benzoate Monobenzoate;Alogliptin API;ALOGLIPTIN(SYK-322) BENZOATE;(R)-2-((6-(3-Aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)benz;2-[[6-[(3R)-3-aMino-1-piperidinyl]-3,4-dihydro-3-Methyl-2,4-dioxo-1(2H)-pyriMidinyl]Methyl]-Benzonitrile Monobenzoate |
| CAS: | 850649-62-6 |
| MF: | C25H27N5O4 |
| MW: | 461.52 |
| EINECS: | 691-730-4 |
| Product Categories: | antidiabetic;Inhibitors;Final material;pharmaceutical intermediate;API |
| Mol File: | 850649-62-6.mol |
| Alogliptin benzoate Chemical Properties |
| Safety Information |
| Alogliptin benzoate Usage And Synthesis |
| Indications and Usage | Alogliptin (benzoic acid) is a type-2 diabetes medication, and it is a type of serine protease dipeptidyl peptidase IV (DPP-4) inhibitor developed by the Japanese company Takeda. Alogliptin, used alone or in combination with other blood sugar-lowering medication, is usually well-tolerated in type-2 diabetes patients. This medication has a low risk of hypoglycemia, with Alogliptin treatment groups ≤8.3% and placebo groups ≤10.5%, and shows no difference between young and elderly patients. In addition to effectively lowering blood sugar, this medicine also lowers the risks of hypoglycemia and weight increase, overcoming great obstacles in patient treatment and providing new hope for diabetes treatment. |
| Mechanisms of Action | Alogliptin selectively inhibits DPP-4 to reduce the inactivation of glucagon-like peptide 1 (GLP-1) and increase the GLP-1 levels in the body, thus lowering blood sugar. Once blood sugar reaches normal levels, it will cease its sugar-lowering effects, thus effectively reducing the risks of hypoglycemia. Additionally, DPP-4 inhibitor also slows gastric emptying, increases the feeling of fullness, and controls appetite, thus helping patients control their weight. |
| Adverse reactions | Common side effects of Alogliptin include nasopharyngitis, headaches, and upper respiratory tract infection. Most side effects are light to moderate and are unrelated to dosage. |
| Uses | Treatment of type 2 diabetes. |
| Definition | ChEBI: A benzoate salt obtained by combining equimolar amounts of alogliptin and benzoic acid. Used for treatment of type 2 diabetes. |
| Clinical Use | Alogliptin benzoate is a dipeptidyl peptidase IV (DPPIV) inhibitor discovered by Takeda Pharmaceuticals and approved in Japan in 2010 for the treatment of type II diabetes mellitus. Alogliptin is an oral drug for once a day dosing to complement diet and exercise. Alogliptin is the most selective marketed DPPIV inhibition and has similar PK and PD properties compared to previous entries. The discovery, structure-activity relationship of related analogs, and synthesis of this compound have been recently published. |
| Chemical Synthesis | The most convenient synthesis for scale-up will be highlighted from several published routes. Commercially available 2-cycanobenzyl amine 1 was reacted with methylisocyanate in DCM at ambient temperature to provide N-methyl urea 2 in 85% yield. Reaction of the urea 2 with dimethyl malonate in refluxing ethanol with sodium ethoxide as base gave the cyclized trione 3 in 78-85% yield. The trione 3 was then refluxed in neat POCl3 to provide the penultimate chloride crude 4 in 95% yield which was reacted with Boc-protected diamine 5 in the presense of potassium carbonate in DMF to furnish alogliptin I in 93-96% yield. Treatment of alogliptin with benzoic acid in ethanol at 60-70 °C followed by crystallization delivered the desired alogliptin benzoate (I).
|
SECTION 2: Hazard identification
2.1 Classification of the substance or mixture
Not classified.
2.2 GHS label elements, including precautionary statements
| Pictogram(s) | No symbol. |
|---|---|
| Signal word | No signal word |
| Hazard statement(s) | none |
| Precautionary statement(s) | |
| Prevention | none |
| Response | none |
| Storage | none |
| Disposal | none |
2.3 Other hazards which do not result in classification
no data available
SECTION 3: Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl] benzonitrile;benzoic acid | 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl] benzonitrile;benzoic acid | 850649-62-6 | 691-730-4 | 100% |
SECTION 4: First-aid measures
4.1 Description of necessary first-aid measures
If inhaled
Move the victim into fresh air. If breathing is difficult, give oxygen. If not breathing, give artificial respiration and consult a doctor immediately. Do not use mouth to mouth resuscitation if the victim ingested or inhaled the chemical.
Following skin contact
Take off contaminated clothing immediately. Wash off with soap and plenty of water. Consult a doctor.
Following eye contact
Rinse with pure water for at least 15 minutes. Consult a doctor.
Following ingestion
Rinse mouth with water. Do not induce vomiting. Never give anything by mouth to an unconscious person. Call a doctor or Poison Control Center immediately.
4.2 Most important symptoms/effects, acute and delayed
no data available
4.3 Indication of immediate medical attention and special treatment needed, if necessary
no data available
SECTION 5: Fire-fighting measures
5.1 Suitable extinguishing media
Use dry chemical, carbon dioxide or alcohol-resistant foam.
5.2 Specific hazards arising from the chemical
no data available
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
SECTION 6: Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing mist, gas or vapours.Avoid contacting with skin and eye. Use personal protective equipment.Wear chemical impermeable gloves. Ensure adequate ventilation.Remove all sources of ignition. Evacuate personnel to safe areas.Keep people away from and upwind of spill/leak.
6.2 Environmental precautions
Prevent further spillage or leakage if it is safe to do so. Do not let the chemical enter drains. Discharge into the environment must be avoided.
6.3 Methods and materials for containment and cleaning up
Collect and arrange disposal. Keep the chemical in suitable and closed containers for disposal. Remove all sources of ignition. Use spark-proof tools and explosion-proof equipment. Adhered or collected material should be promptly disposed of, in accordance with appropriate laws and regulations.
SECTION 7: Handling and storage
7.1 Precautions for safe handling
Handling in a well ventilated place. Wear suitable protective clothing. Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Use non-sparking tools. Prevent fire caused by electrostatic discharge steam.
7.2 Conditions for safe storage, including any incompatibilities
Store the container tightly closed in a dry, cool and well-ventilated place. Store apart from foodstuff containers or incompatible materials.
SECTION 8: Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
no data available
Biological limit values
no data available
8.2 Appropriate engineering controls
Ensure adequate ventilation. Handle in accordance with good industrial hygiene and safety practice. Set up emergency exits and the risk-elimination area.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Wear tightly fitting safety goggles with side-shields conforming to EN 166(EU) or NIOSH (US).
Skin protection
Wear fire/flame resistant and impervious clothing. Handle with gloves. Gloves must be inspected prior to use. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
If the exposure limits are exceeded, irritation or other symptoms are experienced, use a full-face respirator.
Thermal hazards
no data available
